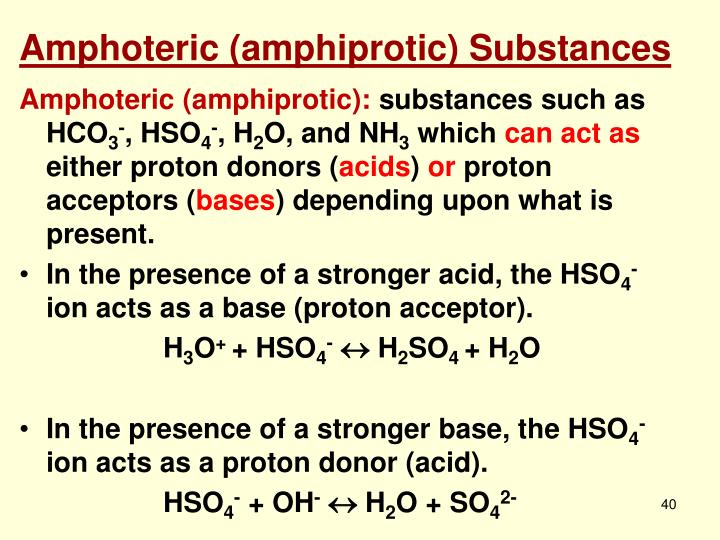
Amino Acid Amphiprotic Examples . Amino acids, the building blocks of. Water can donate its proton {eq}h^+. water, amino acids, hydrogencarbonate ion (or bicarbonate ion) hco− 3, dihydrogen phosphate ion h2po− 4, and. Amino acids such as glycine are amphoteric. A molecule that is both an acid and a base. in biochemistry are widely spread example of amphoteric molecules are proteinogenic amino acids, since they have at least an. An amphiprotic substance is one that can both donate and accept a proton (h+ ion) in a chemical reaction. amphoteric substances are chemical substances able to act as both acids and bases. Metal oxides or hydroxides are amphoteric. The carboxyl group is able to lose a proton and. in addition, amino acids are amphiprotic; They can react either as acids or as bases, depending on the circumstances. in addition, amino acids are amphiprotic; If it gains a hydrogen atoms. In an acidic environment, water can act as a base by accepting a proton,.
from www.slideserve.com
It can act as an acid by donating a. in addition, amino acids are amphiprotic; If it gains a hydrogen atoms. in addition, amino acids are amphiprotic; Water can donate its proton {eq}h^+. Amino acids, the building blocks of. amphoteric substances are chemical substances able to act as both acids and bases. Amino acids such as glycine are amphoteric. in addition, amino acids are amphiprotic; They can react either as acids or as bases, depending on the circumstances.
PPT Acids and Bases PowerPoint Presentation ID3842068
Amino Acid Amphiprotic Examples It can act as an acid by donating a. amphoteric substances are chemical substances able to act as both acids and bases. Amino acids, the building blocks of. If it gains a hydrogen atoms. in addition, amino acids are amphiprotic; In fact the amino acids usually exist in zwitterion (german for “double ion”) form, where the proton has transferred from the carboxyl to the amino group. in addition, amino acids are amphiprotic; here are some examples of amphoterism: examples of amphiprotic molecules include amino acids, proteins, and water. Water (h2o) is a very common solvent and an amphiprotic species. They can react either as acids or as bases, depending on the circumstances. in addition, amino acids are amphiprotic; Amino acids such as glycine are amphoteric. The carboxyl group is able to lose a proton and. It can act as an acid by donating a. in biochemistry are widely spread example of amphoteric molecules are proteinogenic amino acids, since they have at least an.
From www.slideserve.com
PPT SCH4U Unit 2 EQUILIBRIUM PowerPoint Presentation ID3432915 Amino Acid Amphiprotic Examples Amino acids such as glycine are amphoteric. Each amino acid molecule contains an acidic carboxyl group and a basic amino group. in addition, amino acids are amphiprotic; An amphiprotic species is a type of amphoteric substances that either accepts or donates a proton (h. another important group of amphiprotic species is the amino acids. The carboxyl group is. Amino Acid Amphiprotic Examples.
From www.youtube.com
Amphiprotic Substances // HSC Chemistry YouTube Amino Acid Amphiprotic Examples in biochemistry are widely spread example of amphoteric molecules are proteinogenic amino acids, since they have at least an. It can act as an acid by donating a. An amphiprotic substance is one that can both donate and accept a proton (h+ ion) in a chemical reaction. in addition, amino acids are amphiprotic; here are some examples. Amino Acid Amphiprotic Examples.
From slideplayer.com
Chapter 17 Acids and Bases ppt download Amino Acid Amphiprotic Examples The carboxyl group is able to lose a proton and. A molecule that is both an acid and a base. here are some examples of amphoterism: Amino acids, the building blocks of. one example of an amphiprotic substance is water (h2o). In an acidic environment, water can act as a base by accepting a proton,. Water (h2o) is. Amino Acid Amphiprotic Examples.
From www.youtube.com
Amino Acid as Zwitterion, Amphiprotic substance Amphoteric Amino Acid Amphiprotic Examples examples of amphiprotic molecules include amino acids, proteins, and water. water, amino acids, hydrogen carbonate ions and hydrogen sulfate ions are common examples of amphiprotic species. an amphiprotic compound has both acidic and basic properties and can act as either as an acid or a base since an. in addition, amino acids are amphiprotic; An amphiprotic. Amino Acid Amphiprotic Examples.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6416259 Amino Acid Amphiprotic Examples in addition, amino acids are amphiprotic; They can react either as acids or as bases, depending on the circumstances. amphoteric substances are chemical substances able to act as both acids and bases. water, amino acids, hydrogen carbonate ions and hydrogen sulfate ions are common examples of amphiprotic species. Metal oxides or hydroxides are amphoteric. an amphiprotic. Amino Acid Amphiprotic Examples.
From www.slideserve.com
PPT Topic 8 Acids and Bases PowerPoint Presentation, free download Amino Acid Amphiprotic Examples An amphiprotic species is a type of amphoteric substances that either accepts or donates a proton (h. They can react either as acids or as bases, depending on the circumstances. An amphiprotic substance is one that can both donate and accept a proton (h+ ion) in a chemical reaction. water, amino acids, hydrogen carbonate ions and hydrogen sulfate ions. Amino Acid Amphiprotic Examples.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID546616 Amino Acid Amphiprotic Examples In an acidic environment, water can act as a base by accepting a proton,. It can act as an acid by donating a. water, amino acids, hydrogen carbonate ions and hydrogen sulfate ions are common examples of amphiprotic species. in addition, amino acids are amphiprotic; Amino acids, the building blocks of. Metal oxides or hydroxides are amphoteric. Each. Amino Acid Amphiprotic Examples.
From www.youtube.com
8.1 Amphiprotic species (SL) YouTube Amino Acid Amphiprotic Examples Water (h2o) is a very common solvent and an amphiprotic species. here are some examples of amphoterism: An amphiprotic substance is one that can both donate and accept a proton (h+ ion) in a chemical reaction. in addition, amino acids are amphiprotic; in addition, amino acids are amphiprotic; They can react either as acids or as bases,. Amino Acid Amphiprotic Examples.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation ID3842068 Amino Acid Amphiprotic Examples They can react either as acids or as bases, depending on the circumstances. The carboxyl group is able to lose a proton and. In aqueous acid solution, an amino. water, amino acids, hydrogencarbonate ion (or bicarbonate ion) hco− 3, dihydrogen phosphate ion h2po− 4, and. in biochemistry are widely spread example of amphoteric molecules are proteinogenic amino acids,. Amino Acid Amphiprotic Examples.
From www.expii.com
Amphiprotic vs. Amphoteric — Comparison & Examples Expii Amino Acid Amphiprotic Examples examples of amphiprotic molecules include amino acids, proteins, and water. In an acidic environment, water can act as a base by accepting a proton,. in addition, amino acids are amphiprotic; The carboxyl group is able to lose a proton and. water, amino acids, hydrogencarbonate ion (or bicarbonate ion) hco− 3, dihydrogen phosphate ion h2po− 4, and. They. Amino Acid Amphiprotic Examples.
From www.studocu.com
Biochemistry notes Amino Acids are Amphiprotic Molecules Amphiprotic Amino Acid Amphiprotic Examples Amino acids such as glycine are amphoteric. Metal oxides or hydroxides are amphoteric. Each amino acid molecule contains an acidic carboxyl group and a basic amino group. In fact the amino acids usually exist in zwitterion (german for “double ion”) form, where the proton has transferred from the carboxyl to the amino group. here are some examples of amphoterism:. Amino Acid Amphiprotic Examples.
From pediaa.com
Difference Between Amphiprotic and Amphoteric Amino Acid Amphiprotic Examples in addition, amino acids are amphiprotic; water, amino acids, hydrogen carbonate ions and hydrogen sulfate ions are common examples of amphiprotic species. Amino acids, the building blocks of. If it gains a hydrogen atoms. They can react either as acids or as bases, depending on the circumstances. common examples of amphiprotic species include amino acids. They can. Amino Acid Amphiprotic Examples.
From dxohtncvt.blob.core.windows.net
Amino Acids And Uses at Shelia Cole blog Amino Acid Amphiprotic Examples If it gains a hydrogen atoms. In fact the amino acids usually exist in zwitterion (german for “double ion”) form, where the proton has transferred from the carboxyl to the amino group. an amphiprotic compound has both acidic and basic properties and can act as either as an acid or a base since an. An amphiprotic substance is one. Amino Acid Amphiprotic Examples.
From slideplayer.com
THE CHEMISTRY OF ACIDS AND BASES ppt download Amino Acid Amphiprotic Examples An amphiprotic species is a type of amphoteric substances that either accepts or donates a proton (h. Water (h2o) is a very common solvent and an amphiprotic species. common examples of amphiprotic species include amino acids. amino acids are amphoteric, which means they have acidic and basic tendencies. examples of amphiprotic molecules include amino acids, proteins, and. Amino Acid Amphiprotic Examples.
From www.researchgate.net
4. Two amino acids (the carboxylicgroup from the amino acid 1 and the Amino Acid Amphiprotic Examples water, amino acids, hydrogen carbonate ions and hydrogen sulfate ions are common examples of amphiprotic species. The carboxyl group is able to lose a proton and. An amphiprotic substance is one that can both donate and accept a proton (h+ ion) in a chemical reaction. water, amino acids, hydrogencarbonate ion (or bicarbonate ion) hco− 3, dihydrogen phosphate ion. Amino Acid Amphiprotic Examples.
From www.youtube.com
R3.1.3 Amphiprotic species YouTube Amino Acid Amphiprotic Examples An amphiprotic substance is one that can both donate and accept a proton (h+ ion) in a chemical reaction. A molecule that is both an acid and a base. The carboxyl group is able to lose a proton and. amino acids are amphoteric, which means they have acidic and basic tendencies. one example of an amphiprotic substance is. Amino Acid Amphiprotic Examples.
From pediaa.com
Difference Between Amphiprotic and Amphoteric Amino Acid Amphiprotic Examples Amino acids such as glycine are amphoteric. examples of amphiprotic molecules include amino acids, proteins, and water. another important group of amphiprotic species is the amino acids. in biochemistry are widely spread example of amphoteric molecules are proteinogenic amino acids, since they have at least an. Each amino acid molecule contains an acidic carboxyl group and a. Amino Acid Amphiprotic Examples.
From www.thoughtco.com
Amphiprotic Definition in Chemistry Amino Acid Amphiprotic Examples amphoteric substances are chemical substances able to act as both acids and bases. Amino acids such as glycine are amphoteric. common examples of amphiprotic species include amino acids. In fact the amino acids usually exist in zwitterion (german for “double ion”) form, where the proton has transferred from the carboxyl to the amino group. in addition, amino. Amino Acid Amphiprotic Examples.